VIDEOS
NOTES

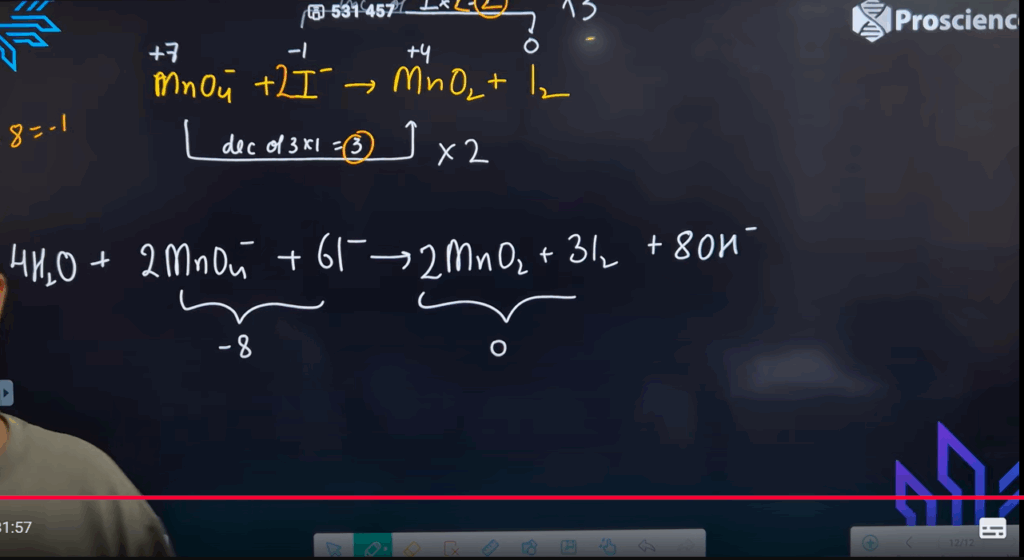
ASSIGNMENT
- Define oxidation number and how it differs from valency.
- What are the basic steps followed in the oxidation number method of redox balancing?
- How does the medium (acidic/basic) affect the balancing of redox reactions?
- Assign oxidation numbers to all elements in the compound: K₂Cr₂O₇
- Identify whether the following is a redox reaction and justify:
CuO(s) + H₂(g) → Cu(s) + H₂O(g)
Section B: Short Answer Questions (2 Marks Each)
- Balance the following redox reaction using the oxidation number method:
Fe²⁺ + Cr₂O₇²⁻ + H⁺ → Fe³⁺ + Cr³⁺ + H₂O - In the reaction: MnO₄⁻ + SO₃²⁻ → Mn²⁺ + SO₄²⁻ (acidic medium),
identify the oxidation number changes and name the oxidising and reducing agents. - Balance the following redox reaction using the oxidation number method (basic medium):
MnO₄⁻ + I⁻ → MnO₂ + I₂ - Explain why oxidation and reduction must occur together in a redox reaction.
- Assign oxidation numbers to sulphur in the following:
a) H₂S
b) H₂SO₄
c) SO₃
d) Na₂S₂O₃
Section C: Long Answer/Problem-Based Questions (3 Marks Each)
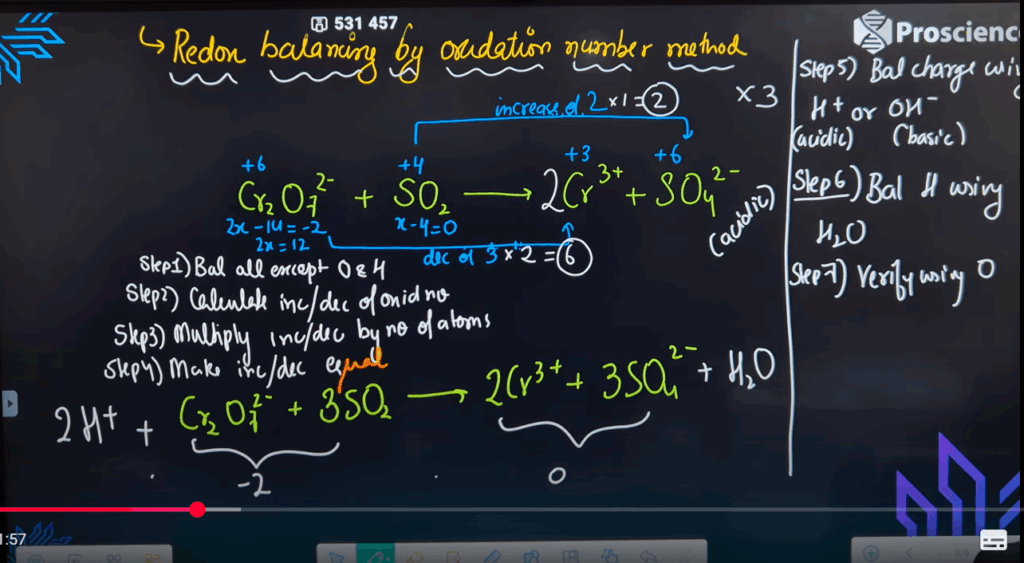
- Balance the redox reaction using the oxidation number method (acidic medium):
Cr₂O₇²⁻ + SO₃²⁻ → Cr³⁺ + SO₄²⁻ - Balance the reaction in basic medium using the oxidation number method:
MnO₄⁻ + Br⁻ → MnO₂ + BrO₃⁻ - A reaction is written as:
Cl₂ + NaOH → NaCl + NaClO + H₂O
a) Assign oxidation numbers.
b) Identify oxidised and reduced species.
c) Balance the reaction using oxidation number method (basic medium). - Explain the role of H⁺ and OH⁻ ions in balancing redox equations using oxidation number method. Provide one example for each case.
- What are the key differences between the half-reaction method and the oxidation number method of redox balancing? State with example.
KEY
Section A: Very Short Answer Questions
- Oxidation number is the charge assigned to an atom in a compound assuming that electrons are completely transferred.
It differs from valency, which is the combining capacity of an atom. - Basic steps:
- Assign oxidation numbers.
- Identify oxidized and reduced elements.
- Balance change in oxidation numbers.
- Balance atoms other than H and O.
- Balance O with H₂O, H with H⁺ (acidic) or OH⁻ (basic).
- Ensure charge and atoms are balanced.
- In acidic medium, use H⁺ and H₂O to balance H and O.
In basic medium, use OH⁻ and H₂O. - K₂Cr₂O₇:
K = +1, O = –2
Total charge = 2(+1) + 2(x) + 7(–2) = 0 → x = +6
Cr = +6 - Yes, it is a redox reaction:
Cu: +2 → 0 (reduction),
H: 0 → +1 (oxidation)
Section B: Short Answer Questions
- Balance (acidic medium):
Fe²⁺ + Cr₂O₇²⁻ + H⁺ → Fe³⁺ + Cr³⁺ + H₂O
Final balanced equation:
6Fe²⁺ + Cr₂O₇²⁻ + 14H⁺ → 6Fe³⁺ + 2Cr³⁺ + 7H₂O - Mn: +7 → +2 (reduction)
S: +4 → +6 (oxidation)
Oxidising agent: MnO₄⁻
Reducing agent: SO₃²⁻ - Balanced (basic medium):
2MnO₄⁻ + I⁻ + 2H₂O → 2MnO₂ + I₂ + 4OH⁻ - Because electron loss (oxidation) must be accompanied by electron gain (reduction), to conserve charge.
- a) H₂S: S = –2
b) H₂SO₄: S = +6
c) SO₃: S = +6
d) Na₂S₂O₃: One S = +6, other = –2 (average +2)
Section C: Long Answer/Problem-Based Questions
- Cr₂O₇²⁻ + SO₃²⁻ → Cr³⁺ + SO₄²⁻ (acidic medium)
Balanced:
Cr₂O₇²⁻ + 3SO₃²⁻ + 8H⁺ → 2Cr³⁺ + 3SO₄²⁻ + 4H₂O - MnO₄⁻ + Br⁻ → MnO₂ + BrO₃⁻ (basic medium)
Balanced:
2MnO₄⁻ + Br⁻ + 2H₂O → 2MnO₂ + BrO₃⁻ + 4OH⁻
a) Cl₂: 0; Cl⁻: –1; ClO⁻: +1
b) Cl₂ is both oxidised and reduced (disproportionation)
c) Balanced:
Cl₂ + 2NaOH → NaCl + NaClO + H₂O
Acidic example:
MnO₄⁻ + Fe²⁺ + H⁺ → Mn²⁺ + Fe³⁺ + H₂O
H⁺ used to balance H atoms
Basic example:
MnO₄⁻ + SO₃²⁻ + OH⁻ → MnO₂ + SO₄²⁻ + H₂O
OH⁻ balances H atoms
| Feature | Half-Reaction Method | Oxidation Number Method |
|---|---|---|
| Basis | Splitting into oxidation/reduction | Changes in oxidation number |
| Medium consideration | More suited to acidic/basic media | Works well with simple redox |
| Electron balancing | Explicit electron transfer shown | Based on change in oxidation states |
| Example: | ||
| Fe²⁺ + Cr₂O₇²⁻ + H⁺ → Fe³⁺ + Cr³⁺ + H₂O (can be done by both methods) |
